Author: Laurence Pendell

- 6 Comments
Learn how to talk to your doctor about staying on a brand-name medication when generics don't work for you. Get practical tips, evidence-based reasons, and steps to fight insurance denials.

- 7 Comments
Endometriosis and interstitial cystitis cause similar pelvic pain, but their treatments differ drastically. Learn how to tell them apart, why misdiagnosis is common, and what steps to take for accurate diagnosis and lasting relief.
- 11 Comments
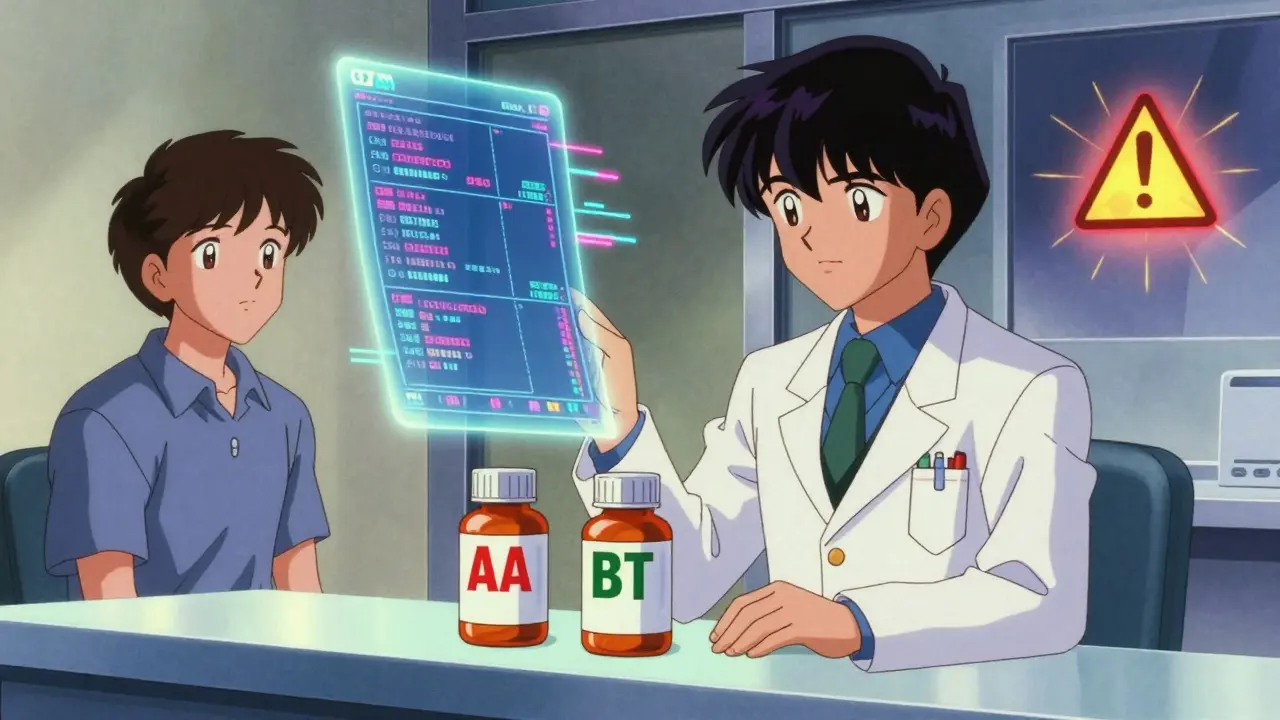
FDA Therapeutic Equivalency (TE) codes determine whether generic drugs can legally be substituted for brand-name drugs. These codes, published in the Orange Book, are enforced by state laws and impact millions of prescriptions annually.
- 12 Comments
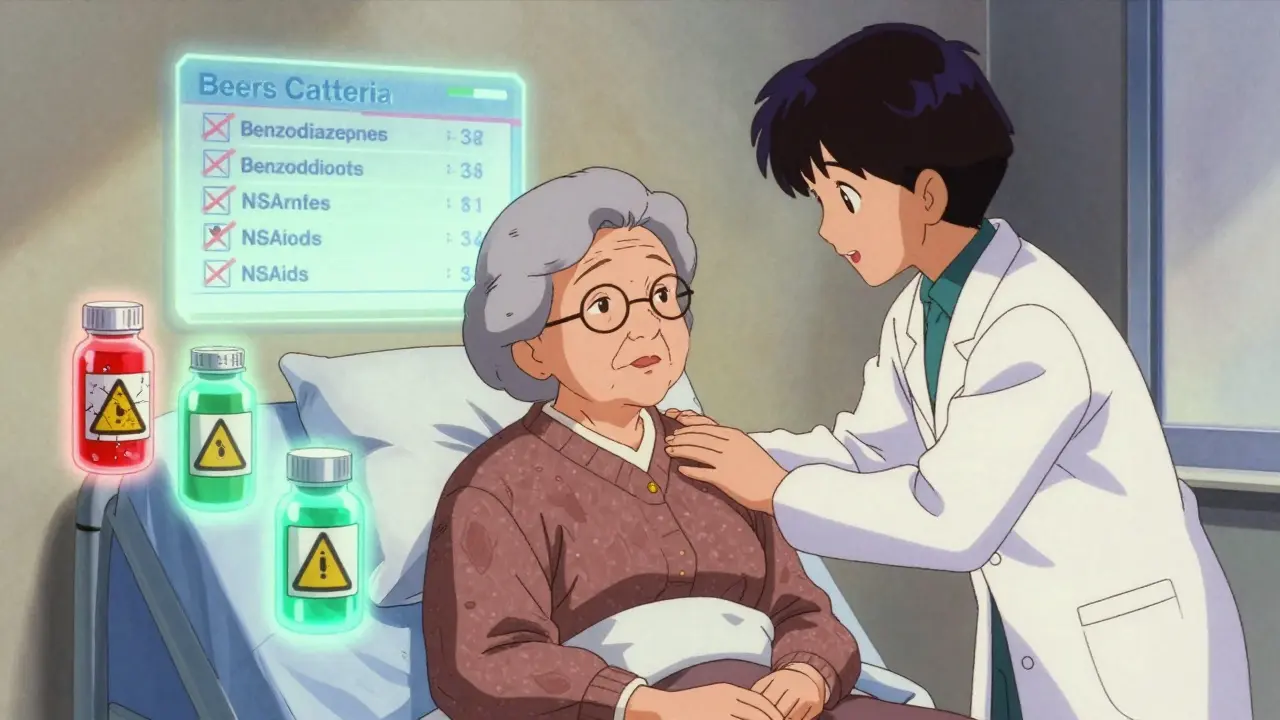
Geriatric medication safety is critical as older adults face higher risks of adverse drug events. Learn how the Beers Criteria and new alternatives help prevent harmful prescribing, reduce hospitalizations, and improve care for seniors.

- 14 Comments
Learn how to prevent accidental poisonings in young children by locking up medications, using proper dosing tools, and avoiding common household hazards. Expert-backed strategies reduce risk by up to 82%.

- 14 Comments
Generics play a crucial role in controlling rising global healthcare costs. With healthcare spending projected to reach $5.6 trillion in the US by 2025, generic drugs offset expensive new medications. Regional data shows varying cost trends, from North America's 8.7% growth to Asia Pacific's 12.3%. Over 55 countries rely on out-of-pocket payments, making generics vital for access. Learn how this balance impacts healthcare worldwide.

- 13 Comments
REM Sleep Behavior Disorder (RBD) involves acting out dreams during sleep, risking injury. Diagnosed via polysomnography. Treatments: melatonin (65%) and clonazepam (85%). Up to 73.5% progress to Parkinson's. Safety tips covered.

- 14 Comments
Learn practical, evidence-based ways to keep taking your medication during life changes like moving, divorce, or job loss. Discover how flexible routines, psychological tools, and simple planning can prevent adherence breakdowns under stress.
- 10 Comments
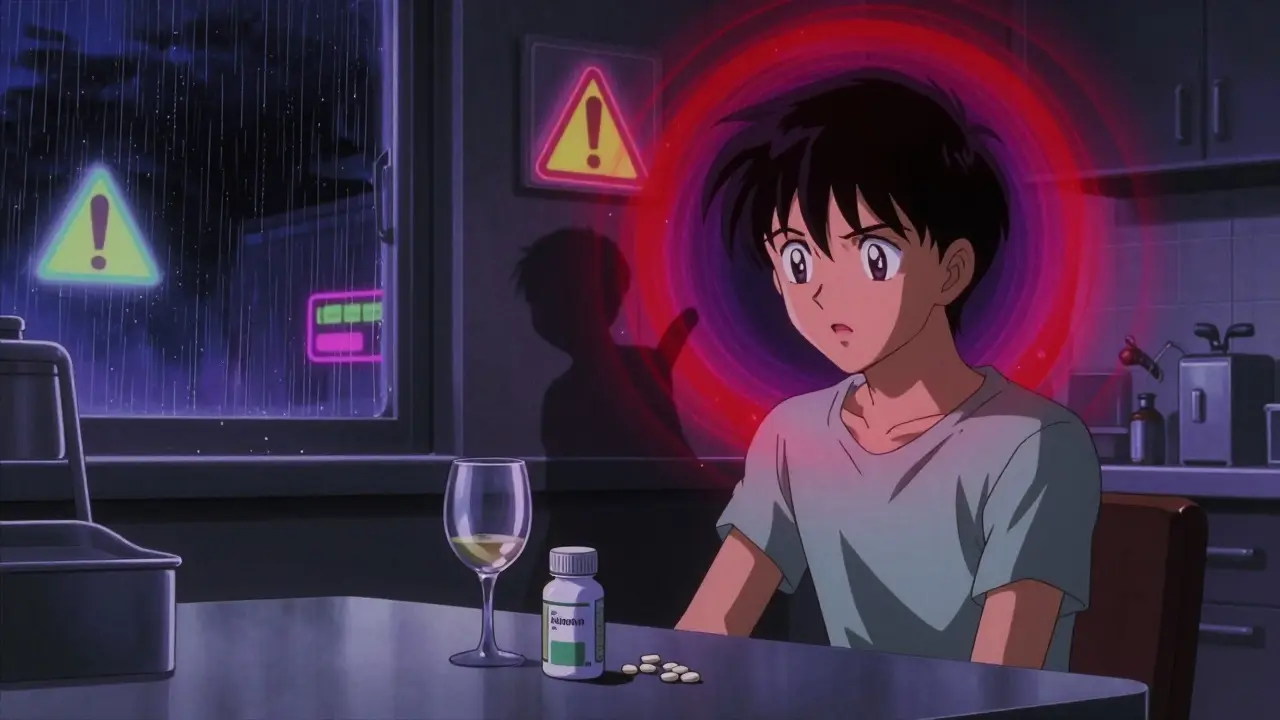
Metformin and alcohol together can trigger lactic acidosis-a rare but deadly condition. Learn the real risks, symptoms to watch for, and what doctors actually recommend if you have type 2 diabetes.
- 10 Comments
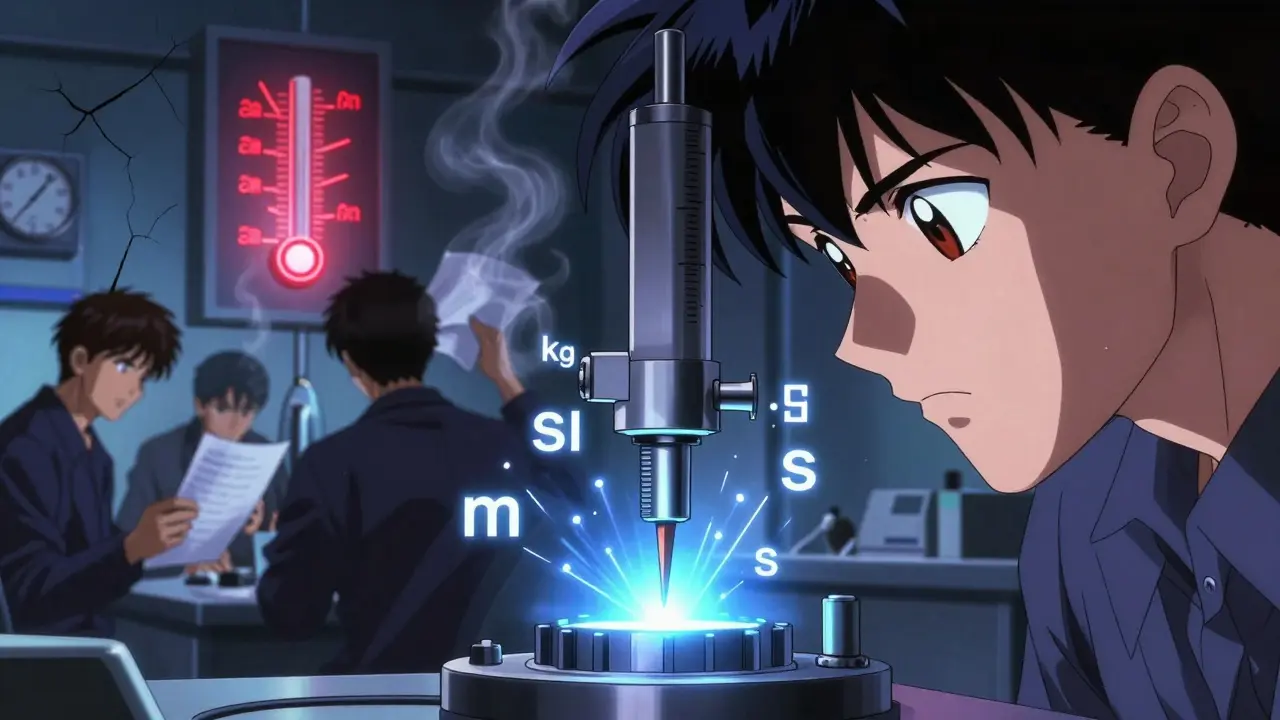
Calibration and validation ensure equipment delivers accurate, reliable measurements in manufacturing. Learn how ISO 13485, FDA, and CLIA requirements shape your process-and how to avoid costly compliance failures.
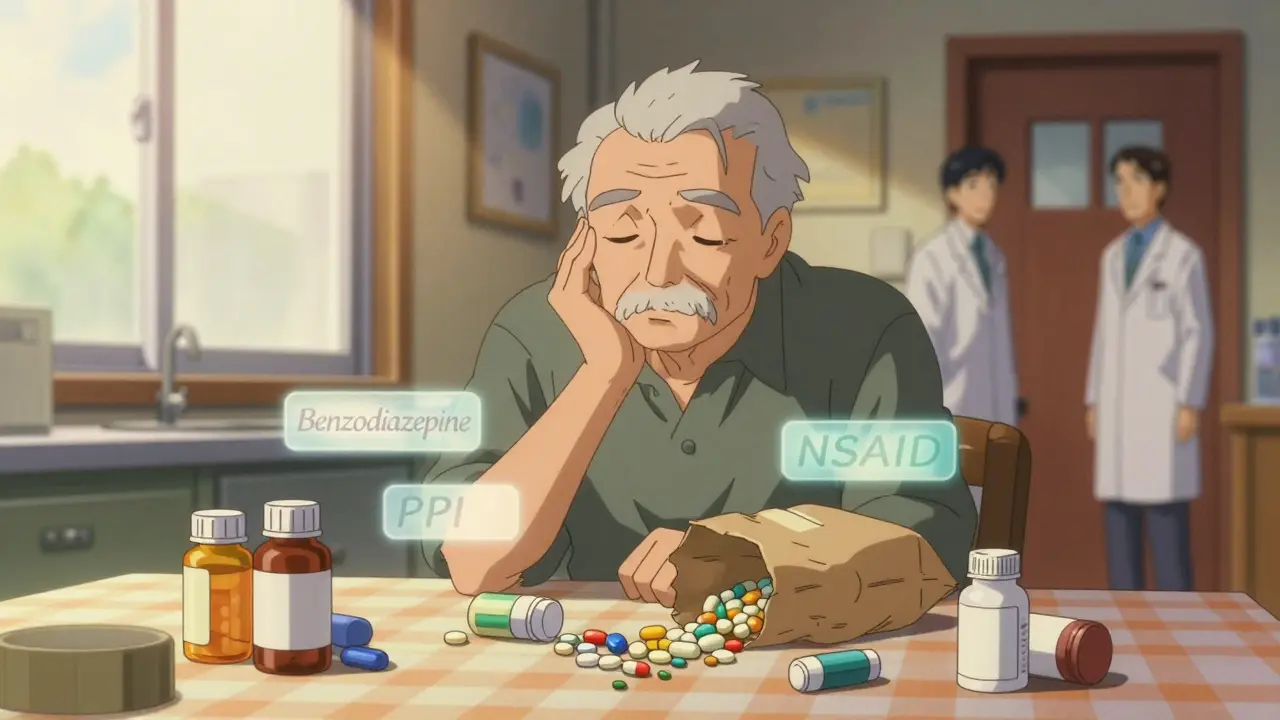
- 14 Comments
Polypharmacy in elderly patients-taking five or more medications-increases risks of falls, confusion, and hospitalization. Learn how deprescribing, medication reviews, and patient advocacy can improve safety and quality of life for seniors.

- 0 Comments
Paragraph IV certifications let generic drug makers challenge brand-name patents before launch, speeding up access to affordable medicines. Learn how the Hatch-Waxman Act enables this system and why it saves billions.