Every time you take a pill, there’s a hidden calculation happening - one that balances the chance it will help you against the chance it might hurt you. This isn’t guesswork. It’s science. And that science is called medication safety.
Why Medication Safety Isn’t Just About Avoiding Mistakes
Most people think medication safety means not mixing pills or not taking the wrong dose. That’s part of it. But the real science goes deeper. It asks: How do we know this drug is truly safe for millions of people - not just the few thousand who tried it in a clinical trial? Clinical trials are tightly controlled. Participants are carefully selected. They’re monitored closely. But real life? People take multiple drugs. They forget doses. They have other health problems. They buy over-the-counter meds without telling their doctor. That’s where clinical trial data falls short. Take the case of Vioxx. It was approved after trials involving about 5,000 people. It worked great for arthritis pain. But after it hit the market and millions started using it, researchers found it doubled the risk of heart attacks. That’s a problem you can’t see in a trial of 5,000 - unless it happens in 1 out of every 10,000 people. And that’s exactly what happened. This is why we need more than trials. We need systems that watch what happens when drugs are used by real people, every day, across entire populations.The Tools That Watch What Drugs Do in the Real World
The main tool for this job is called pharmacoepidemiology. It’s basically epidemiology - the science of tracking disease patterns - but focused on drugs. Instead of studying who gets the flu, it studies who gets a liver injury after taking a certain painkiller. Researchers use massive databases to do this. In the U.S., the FDA’s Sentinel Initiative tracks over 190 million people through insurance claims, hospital records, and pharmacy data. Kaiser Permanente’s system covers 12.5 million members. Medicare tracks 57 million seniors. These aren’t small samples. These are entire populations. They use three main study designs:- Cohort studies: Track groups of people who took a drug vs. those who didn’t, and see who ends up in the hospital.
- Case-control studies: Look at people who had a bad reaction and find out what drugs they took - then compare them to people who didn’t have the reaction.
- Self-controlled designs: Compare what happens to the same person before and after taking a drug. If someone has a seizure right after starting a new seizure medication, it’s more likely the drug caused it - because it’s the same person, with the same body, same lifestyle, same risks.
The Gold Standard Isn’t Always the Best Tool
Randomized controlled trials (RCTs) are called the gold standard. And for good reason. They’re the only way to prove a drug causes an effect - not just that the two things happen together. But here’s the catch: RCTs are expensive and slow. The average Phase III trial costs $26 million and includes only 707 people. They last 6 to 24 months. That’s fine for spotting common side effects - nausea, dizziness, headaches. But not for rare ones. Or long-term ones. Or ones that only show up in older people with kidney disease. That’s where observational studies shine. They cost a fraction - around $150,000 to $500,000 - and can include hundreds of thousands of people over years. They’re not as clean as RCTs, but they’re far more realistic. A 2021 review in JAMA Internal Medicine found that 22% of the strong links found in observational studies were later proven wrong by RCTs. But here’s the flip side: 78% were confirmed. And many of the most important safety signals - like the link between certain antibiotics and heart rhythm problems - were first spotted in observational data. So it’s not about choosing one over the other. It’s about using both. RCTs tell us what a drug can do under ideal conditions. Observational studies tell us what it actually does in the messy world of real patients.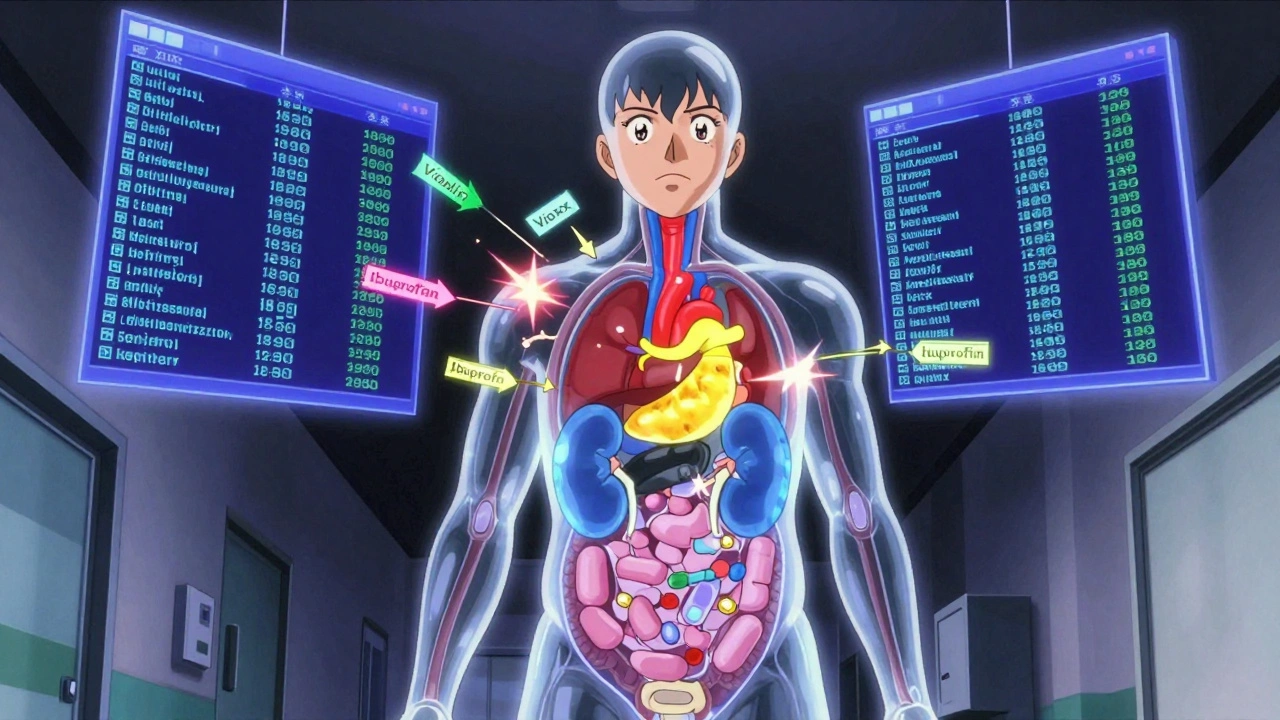
Who’s Watching? Who’s Responsible?
The U.S. Food and Drug Administration (FDA) is the main player. Since the 2007 FDA Amendments Act, the agency can require drugmakers to run post-market safety studies - called Risk Evaluation and Mitigation Strategies (REMS) - for drugs with known dangers. About 37% of new drugs approved between 2015 and 2020 came with these requirements. The European Medicines Agency does the same. And in the U.S., agencies like the National Institutes of Health (NIH) and the Patient-Centered Outcomes Research Institute (PCORI) fund independent research to fill gaps. But hospitals and pharmacies are on the front lines. As of 2023, 63% of U.S. hospitals with over 300 beds have a dedicated medication safety officer. Smaller clinics? Only 28% do. That’s a gap. Electronic health records (EHRs) now have built-in alerts - warning doctors about drug interactions, allergies, or wrong doses. But here’s the problem: doctors get so many alerts that they start ignoring them. One study found that 89% of drug interaction alerts are overridden - especially for common meds like antibiotics or blood pressure pills. It’s alert fatigue. And it’s dangerous.Where It’s Working - And Where It’s Failing
Some places are getting it right. At Kaiser Permanente Washington, they created a standardized protocol for treating alcohol withdrawal with phenobarbital. Before? 15.3% of patients had severe seizures or delirium. After? It dropped to 8.9%. That’s a 42% reduction - just by changing how they gave a drug. In Iran, a study of 500 nurses found that their level of medication safety training directly predicted how many errors happened on their units. Nurses with better training had 61% fewer near-misses. But the problems are still widespread. Older adults are the biggest risk group. Fifteen percent of Medicare patients have a dangerous drug reaction every year. Why? Because they’re often on five or more medications. And the more pills you take, the more chances for interactions. Opioids are another crisis. In 2022, over 80,000 people in the U.S. died from opioid overdoses. Many started with a prescription. That’s why doctors now use tools like prescription drug monitoring programs (PDMPs) - but not everyone uses them consistently. And nursing errors? They account for 38% of all preventable drug mistakes. Not because nurses are careless. Because systems are broken. Fragmented EHRs. Poor communication between shifts. No time to double-check.
What’s Next? AI, Wearables, and Standardization
The field is moving fast. The FDA just launched Sentinel System 3.0 - a faster, smarter way to spot safety signals in real time. Researchers are now using artificial intelligence to predict who’s most likely to have a bad reaction before it happens. Early results show a 22-35% drop in high-alert medication errors when AI flags risks before the doctor even writes the script. Soon, data from smartwatches and fitness trackers could be part of safety monitoring. If someone’s heart rate spikes after taking a new drug, that could be a signal - even if they never went to the hospital. But there’s a catch. A 2024 government report found major gaps in monitoring compounded medications - drugs mixed in pharmacies, often for pain or hormones. These aren’t FDA-approved. They’re not tracked well. And they’re growing in use. Another issue: data privacy. A 2023 Supreme Court decision weakened some protections under HIPAA, making it harder for researchers to use patient data without consent. That could slow down safety research. And without standard methods, studies can’t be compared. One team uses one algorithm to find kidney injuries. Another uses a different one. The results don’t match. That’s why the International Society of Pharmacoepidemiology is pushing for global standards - and they’ve got 3,500 members working on it.What You Can Do
You don’t need to be a scientist to help with medication safety. Here’s how:- Keep a full list of everything you take - prescriptions, supplements, OTC meds, even herbal teas. Bring it to every appointment.
- Ask: “What’s this for? What are the risks?” Don’t assume your doctor knows you’re taking something else. Tell them.
- Use one pharmacy if you can. That way, your pharmacist can spot interactions you might miss.
- Know your high-risk meds - blood thinners, insulin, opioids, sedatives. These are the ones most likely to cause serious harm if misused.
- Report side effects. If you have a strange reaction, tell your doctor - and file a report with the FDA’s MedWatch program. Your report could help save someone else’s life.
The Bottom Line
Medication safety isn’t about eliminating all risk. That’s impossible. It’s about understanding risk - and making sure the benefits outweigh it. It’s about knowing that a drug that helps 90% of people might harm 1 in 10,000. And that 1 person matters. It’s about using real-world data to catch what trials miss. It’s about fixing broken systems so nurses aren’t overwhelmed and doctors aren’t blinded by alerts. And it’s about you - asking questions, staying informed, and speaking up when something doesn’t feel right. Because in the end, medication safety isn’t just science. It’s a promise - that the pills we take will heal us, not hurt us.What’s the difference between a clinical trial and real-world evidence?
Clinical trials test drugs in small, controlled groups under ideal conditions - usually 1,500 to 5,000 people over 6 to 24 months. Real-world evidence looks at how drugs behave in millions of people over years, using data from insurance claims, hospitals, and pharmacies. Trials tell you if a drug works in theory. Real-world evidence tells you if it’s safe in practice.
Can a drug be approved even if it has risks?
Yes. The FDA approves drugs when the benefits clearly outweigh the risks for the intended use. For example, chemotherapy drugs are highly toxic, but they save lives in cancer patients. The key is transparency: the risks must be known, communicated, and monitored - often through post-market safety studies.
Why do doctors ignore drug interaction alerts?
They’re overwhelmed. In emergency rooms, prescribers get dozens of alerts per shift. Many are low-risk or redundant - like warnings for common drug pairs that are safe in most cases. After a while, they start tuning them out. This is called alert fatigue, and it’s a major safety risk. Better, smarter alerts - not more alerts - are the solution.
Are over-the-counter (OTC) drugs safe?
Not always. Many people assume OTC means harmless. But drugs like ibuprofen, acetaminophen, and antihistamines can cause serious harm - especially when mixed with other meds or taken long-term. Acetaminophen overdose is the leading cause of acute liver failure in the U.S. Always check with your pharmacist before combining OTC and prescription drugs.
How can I report a bad reaction to a medication?
You can report it directly to the FDA through their MedWatch program - online, by phone, or by mail. Your report helps identify new safety signals. Even if you’re not sure the drug caused it, report it. The FDA looks for patterns - one report might not mean much, but 50 similar reports can change a drug’s warning label.
Is medication safety getting better or worse?
It’s improving - but slowly. Technology like AI and real-time data systems are helping catch risks faster. But problems like polypharmacy in older adults, alert fatigue, and gaps in monitoring compounded drugs are growing. Funding for safety research is rising - up 25% annually through 2030 - which is good news. But real progress needs better systems, not just better tools.

Kyle Flores
December 8, 2025 AT 01:44I’ve been on five meds for years and never realized how much my pharmacist knows that my doctor doesn’t. Just started using one pharmacy and already caught a bad interaction with my blood pressure pill and that herbal stuff I take for sleep. Seriously, if you’re on more than three pills, do yourself a favor and get a pharmacy buddy.
Ryan Sullivan
December 10, 2025 AT 00:28The entire pharmacovigilance infrastructure is a house of cards built on observational bias and regulatory capture. RCTs are the only valid epistemic framework-everything else is post-hoc noise masquerading as science. The FDA’s Sentinel Initiative is a bureaucratic vanity project funded by pharmaceutical lobbying dollars. Real evidence requires randomization, not claims data mining.
Jennifer Anderson
December 10, 2025 AT 22:13My grandma took 8 pills a day and no one ever asked her if she knew why. She’d just say ‘the doctor said so.’ I wish someone had taught her to ask questions like this article says. We need more of this info in plain language for older folks. Maybe community centers could host ‘Medication 101’ nights?
Oliver Damon
December 12, 2025 AT 20:11The epistemological tension between RCTs and real-world evidence reflects a deeper philosophical divide: reductionism versus emergent complexity. Clinical trials isolate variables to establish causality, but they flatten the multidimensional reality of human physiology, comorbidities, and behavioral noncompliance. Observational data, despite its confounders, captures the systemic noise that defines therapeutic outcomes in lived experience. Neither is superior-both are necessary lenses.
Louis Llaine
December 13, 2025 AT 21:35So let me get this straight-doctors ignore 89% of alerts because they’re annoying, but we’re supposed to trust the system? Wow. Next they’ll tell us seatbelts are optional because people forget to buckle up. Thanks for the update, science.
Jane Quitain
December 14, 2025 AT 20:12OMG I just realized I’ve been taking ibuprofen with my blood thinner for years 😳 I’m so glad I read this! I’m going to the pharmacy right now!! You guys are lifesavers!! 💕
Nicholas Heer
December 16, 2025 AT 13:12Big Pharma and the FDA are in bed together. Vioxx? That was a cover-up. They knew. They always know. The real-world data they’re ‘discovering’ now? It’s been buried for decades. And now they want to use AI? That’s just another way to control the narrative. Wake up people-this isn’t safety, it’s surveillance.
Stacy here
December 17, 2025 AT 12:06It’s not just about drugs-it’s about trust. We’re told to believe in science, but then we see the same companies that made opioids get fined billions and still get to sell new drugs. How do you trust a system that lets profit dictate safety? We need to stop treating patients like data points and start treating them like humans. And maybe, just maybe, stop letting corporations write the rules.
David Brooks
December 18, 2025 AT 17:07This is the most important article I’ve read all year. Seriously. If you take ANY medication-even aspirin-read this twice. Share it with your family. We’ve been sleepwalking through our own health. Time to wake up.
Kurt Russell
December 19, 2025 AT 02:28I’m a nurse and I can tell you-alert fatigue is real. We get 30+ pop-ups per shift. Half are for stuff like ‘this patient is on two NSAIDs’ when they’re both low-dose and approved together. We’re not lazy-we’re drowning. Fix the system, not the people.
Wesley Phillips
December 20, 2025 AT 10:55Pharmacoepidemiology is just statistics with a lab coat. You can correlate anything with enough data-ice cream sales and drowning deaths, anyone? The FDA’s obsession with ‘real-world evidence’ is a desperate attempt to justify rushed approvals. RCTs are the gold standard for a reason. Everything else is just correlation theater.
Sam Mathew Cheriyan
December 21, 2025 AT 00:46USA thinks it’s the only one doing this? LOL. India has been tracking drug reactions since 2008 with a national database. We don’t have fancy AI but we have 1.4 billion people and zero patience for corporate BS. Your system is broken. We’re ahead.
Ernie Blevins
December 22, 2025 AT 21:17So what? People die. That’s life. You take a pill, you might die. Big deal. Stop crying about it. The market will fix it. More pills = more profit. That’s capitalism. Deal with it.
Nancy Carlsen
December 23, 2025 AT 11:46Thank you for writing this!! 🙏 I just shared it with my mom and my book club. We’re all going to start keeping med lists and asking questions! And I’m filing a MedWatch report for that weird rash I got last month-just in case. 💪❤️